Scroll to Watch Video &
Learn More

The current COVID-19 environment has created additional challenges in management of myelosuppression
Multiple blood cell lineages break down during chemotherapy
Despite current interventions, patients’ neutrophils, red blood cells, and platelets continue to be depleted due to myelosuppression1
Scroll to learn more about
- The need to reassess the consequences of myelosuppression
- The role of hematopoietic stem and progenitor cells (HSPCs)—the source of all blood cells
Watch a video to hear Dr. Jeffrey Crawford, Medical Oncologist, talk about facing the challenge of myelosuppression in the current environment
HSPCs, the source of all blood cell lineages, continue to be damaged by chemotherapy—leading to multiple consequences
More than neutrophil reduction
Chemotherapy-induced damage to HSPCs leads to depletion of the blood cells they produce, leading to a range of different adverse events that affect patients1
Adverse events can compromise treatment
Chemotherapy dose delays or reductions may be linked to a decrease in overall survival across multiple cancer types2-5
The impact continues
Many interventions for myelosuppression are used with the goal of stimulating or replacing individual cell lineages after damage to HSPCs in the bone marrow and cell depletion occur1
Current healthcare considerations
The current healthcare environment has created additional challenges in myelosuppression management due to limited blood supply, infection risk, and the need to minimize patient exposure, office visits, and hospitalizations
Neutrophils

Neutropenia still affects many patients
- Neutropenia continues to increase patients' risk of infection and results in increased office visits and hospitalizations1
- Neutropenia is one of the top reasons chemotherapy patients enter the emergency room or hospital6
- Patients who had neutropenia during chemotherapy reported lower quality-of-life scores midcycle and after completing therapy7
-
Patients may still experience Grade 3/4 neutropenia even when granulocyte colony-stimulating factors (G-CSFs) are used to attempt to stimulate the neutrophil lineage after damage to HSPCs by chemotherapy1
It is important to monitor patients for neutropenia, even when G-CSFs are given prophylactically, as well as to monitor for potential side effects of G-CSFs such as bone pain.1
Erythrocytes
The impact of anemia may be underestimated
- While neutropenia is often a primary focus, chemotherapy-induced anemia has serious consequences that affect a patient’s quality of life, morbidity, and mortality, as well as healthcare utilization8
- Anemia causes fatigue, shortness of breath, and dizziness and has a significant negative impact on patients’ functional and emotional well-being scores8
-
The need to receive RBC transfusions creates additional burden for patients and impacts patient care9
Patients are required to leave home and travel for appointments and procedures. RBC transfusions also carry risks of immunosuppression, alloimmunization, and transfusion reactions10,11 - Anemia, like neutropenia, is one of the top reasons chemotherapy patients enter the emergency room or hospital6
Platelets

Treatment options for thrombocytopenia
are limited
- There is currently no approved medication for chemotherapy-induced thrombocytopenia1,12
-
When platelet transfusions are needed, they create additional burden for patients; additionally, transfusions may have adverse events, and patients may potentially become refractory13
Adverse events may include febrile and allergic reactions, transfusion-related acute lung injury, and infection due to bacterial contamination13 - In patients with thrombocytopenia, doctors may choose to adjust the dose intensity and schedule of chemotherapy to avoid platelet transfusions1
T-lymphocyte
B-lymphocyte
A patient’s immune system is suppressed
Chemotherapy also results in damage to lymphocyte lineages, including T-cells as well as B-cells14
These cells have been shown to play an important role in the immune system’s response to certain tumor types15
All the blood cell lineages broken down by chemotherapy stem from HSPCs, critical source cells
HSPCs are the source of all blood cell lineages, including neutrophils, red blood cells, and platelets, as well as lymphocytes (T-cells and B-cells)1
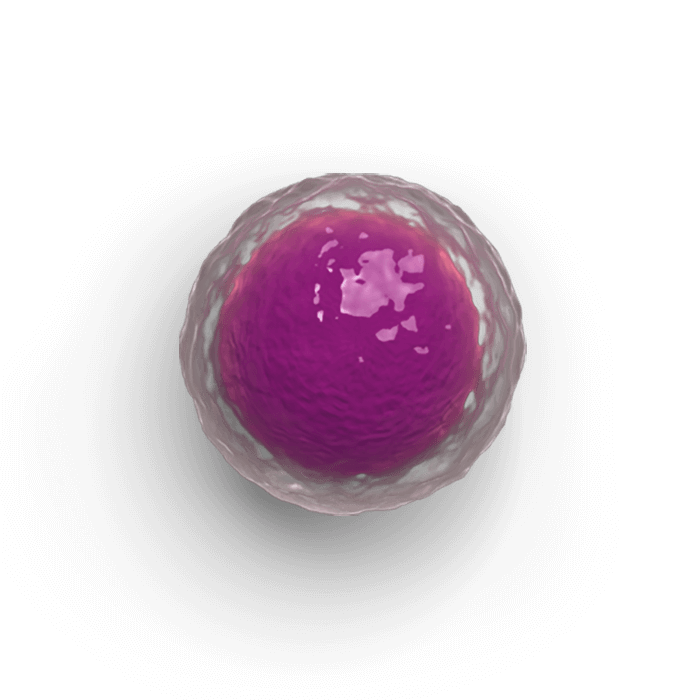
HSPCs
We are exploring chemotherapy-induced myelosuppression at its root—damage to HSPCs, the source of all blood cell lineages
We are committed to evaluating the starting point of neutropenia, anemia, and thrombocytopenia in patients being treated with myelosuppressive chemotherapy.
In what percent of patients do you need to alter the planned treatment course due to myelosuppressive AEs?
Hear Dr. Jeffrey Crawford, Medical Oncologist, share insights about the increasing importance of addressing myelosuppression
HCP/Patient Blood Count Discussion Sheet
This resource is designed to assist healthcare professionals in providing their patients with important information about myelosuppressive risk during chemotherapy and how it can be managed.
Download Info Sheet